Berlin Heart Today Announces CE Approval and First Implantation of an Innovative Bridging Solution for Single Ventricle Patients
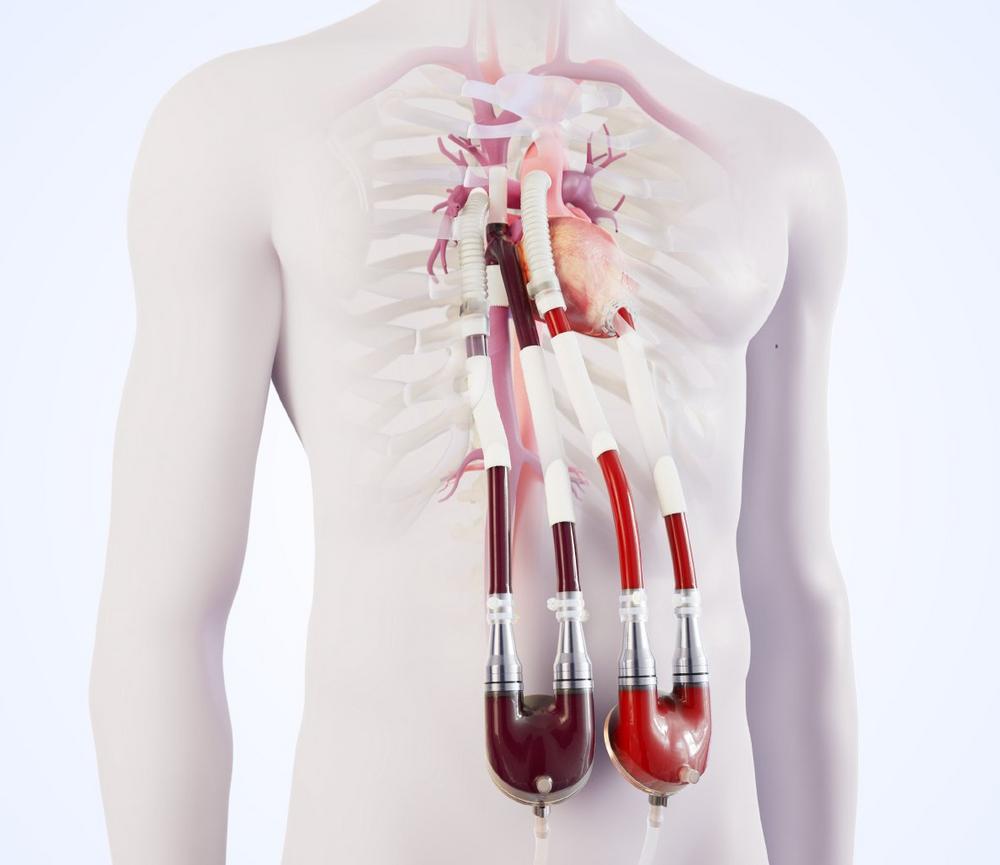
This innovative therapy was developed to improve the end-organ function and hemodynamics of these patients with univentricular physiology through the support of the sub-pulmonary circulation. Thus, the Berlin Heart Venous Cannula builds the bridge to transplant, it builds the bridge to life, for patients with failing Fontan circulation.
Amazing improvements of surgical therapies and patient care ensure that even children with complex congenital heart defects, such as a single ventricle, may survive and live into adulthood.
Nevertheless, for many patients, the time comes, when due to worsening heart failure the only remaining hope is a donor heart. With the aim of giving these patients a chance to bridge the sometimes very long waiting times, Berlin Heart has developed the EXCOR® Venous Cannula.
Based on an idea for a specific venous cannula replacing the right atrium and modified according to the original design input of Dr. Eugen Sandica, Pediatric and Congenital Heart Surgeon from the Heart and Diabetes Centre Bad Oeynhausen, this cannula was specifically developed for the unique challenges of a failing Fontan circulation. The cannula is designed to reduce the complexity of surgery, improve the sub-pulmonary hemodynamics and enable the end-organ recovery.
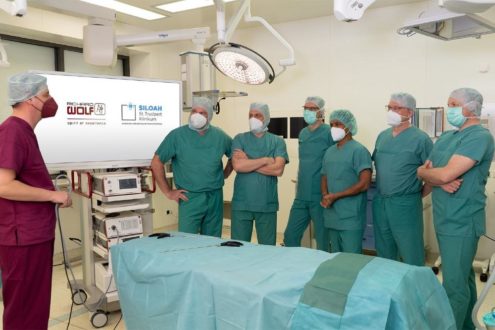
The Pediatric Heart-Team from the LMU University Hospital Munich was the first to use this device. The specialized pediatric cardiac surgical team led by Prof. Juergen Hoerer (Pediatric Heart Surgeon) implanted this cannula. Prof. Nikolaus Haas (Pediatric Cardiologist and Intensivist) emphasises the valuable new option: “These patients often have adequate function of their ventricle but extremely poor circulation due to this unphysiological flow pattern after Fontan surgery. In this situation, artificial sub-pulmonary support is the only way to normalize hemodynamics, allow for organ recovery during mechanical support therapy and consequently has the potential to lower mortality and morbidity on the waiting list. Moreover, we are confident that we can reduce the risk of the heart transplantation in failing Fontan hemodynamics.”
Patients with Fontan circulation suffer from many associated end-organ dysfunctions caused by poor blood circulation like liver failure (Fontan-associated-liver-disease (FALD)), severe problems of the gut with uncontrollable diarrhoea (protein losing enteropathy) or life threatening lung affections (plastic bronchitis). To overcome this, they urgently need a donor heart. While these significant comorbidities indicate a heart transplantation, at the same time, they worsen its chance of success.
Dr. Eugen Sandica highlights the outstanding feature of this new surgical tool: “The specifically designed EXCOR® Venous Cannula facilitates a standardized implantation technique and enables mechanical circulatory support of the sub-pulmonary circulation in patients with failing Fontan for the very first time. This cannula permits the use of the EXCOR® VAD as sub-pulmonary support alone (RVAD) or in combination with a LVAD, in a so called BVAD mode in a majority of patients with failing Fontan circulation, thereby serving an unmet medical need.”
The clinical improvement due to this novel cannula will be assessed within a prospective, international, multi-centre registry study “RegiVe” – Registry to assess the safety and feasibility of the subpulmonary support with the novel Venous Cannula in patients with failing/absence of the right heart. This study will take place at ten experienced centres in five European countries and will collect data on clinical condition, organ function, quality of life and outcomes, with up to a one–year follow-up.
Dr. Ares K. Menon, Cardiac Surgeon and General Manager of Berlin Heart GmbH has a very clear vision: “The EXCOR® Venous Cannula enables our clinical partners to build a bridge to transplant for patients with failing Fontan circulation. Our ambition is to reduce mortality and morbidity on the waiting list and increase the chances of these patients for a successful heart transplantation.“
Berlin Heart’s philosophy is based on future orientation and investment in research and development, thereby always in focus to offer a suitable solution for every single patient.
Another important milestone in the history of Berlin Heart was reached with this new development.
Background
Some children are born with a so called single-ventricle physiology: Instead of a two-chambered heart, they lack one of the ventricles or suffer from an imbalance, resulting in only one functional beating heart chamber (ventricle). In several operations the systemic (left) and pulmonary (right)-sided circulation are gradually separated to establish a stable body and pulmonary circulation. The last step is the so-called Fontan operation named after the French heart surgeon and pioneer Dr. Francis Fontan. Although this operation is live saving, it remains a palliation. Due to this unphysiological blood circulation, all Fontan patients experience a worsening of their heart and other organ function over time, a so called failing of their Fontan circulation.
Please read more about: https://www.berlinheart.de/en/medical-professionals/excorr-venous-cannula/
Berlin Heart GmbH develops, manufactures, and markets innovative ventricular assist devices (VADs) for mechanical circulatory support. With the EXCOR® Adult and the EXCOR® Pediatric, Berlin Heart is the only company in the world able to provide support to patients of every age and size, from infants to adults, thereby meeting an important worldwide unmet need.
Berlin Heart’s systems provide left ventricular, right ventricular, or biventricular support (both sides); hospitals around the world use the system for short-, medium-, and long-term circulatory support, providing patients with a life-saving treatment option.
Users can also access round-the-clock medical and technical support.
The access to some or all shown products may be restricted by country-specific regulatory approvals. The use of EXCOR® VAD for adults, RVAD-support, EXCOR® Venous Cannula, EXCOR® Arterial Cannula for Graft, Excor mobile and EXCOR® Active is not FDA approved and not available for commercial use in the US. The configuration of the EXCOR® Arterial Cannula for Graft as shown in the figure has not yet been CE marked.
Berlin Heart GmbH
Wiesenweg 10
12247 Berlin
Telefon: +49 (30) 8187-2600
Telefax: +49 (30) 8187-2601
http://www.berlinheart.de
Marketing & Public Relations
E-Mail: theresa.tolle@berlinheart.de
![]()