Order for Gerresheimer
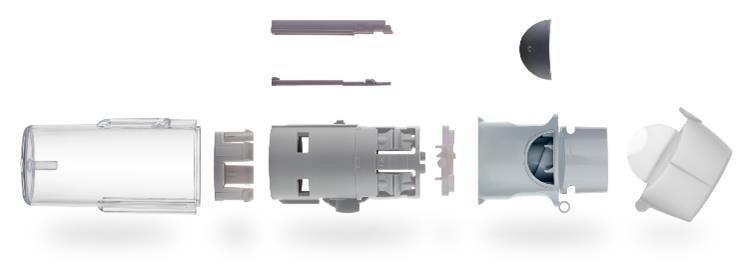
The Respimat® is an inhaler for the treatment of respiratory diseases that is firmly established in the market. Patients with chronic lung diseases like COPD use bronchodilation drugs on a daily basis to relieve their illnesses. Correspondingly high is the consumption of inhalers, which usually need to be replaced when the active agent has been exhausted. Boehringer Ingelheim has therefore decided to develop a new, reusable version of the Respimat®. This further development of the inhaler takes the feedback of patients into account. Thus, with a view to the ergonomics of the Respimat®, the grip has been further improved by an extension of the housing. The readability of the dosage display has also been improved.
Challenging for product development and industrialization was the necessity the new inhaler be immediately available in large numbers for its market launch. Gerresheimer, therefore, had to immediately transition from the development phase to a robust, high-volume, series production. In order to meet such a demanding schedule, the development phase and the creation of the equipment for large series production were advanced simultaneously. The foundation was first established with low-cavitiy molds and semi-automated processes, on the basis of which the development of high-cavitiy molds and completely automated processes for high-volume, large series production were immediately commenced with. In this way, the development of the series equipment be initiated 10 months prior to the planned design verification. Also decisive for the success of the project was the availability of our own clean room production for small series, with which samples could be promptly tested under real conditions.
A risk-based approach that ensures the systematic mastering of all risks of the production process was used for the jump to large series production. Due to this robust development approach, all functional tests for the design verification of the low-cavity molds and later for the implementation of the high-cavitiy series molds were passed immediately. High-volume series production has also been running for several months now without problems.
Gerresheimer is a leading global partner to the pharma and healthcare industry. With specialty glass and plastic products, the Company contributes to health and well-being. Gerresheimer operates worldwide and its approximately 10,000 employees manufacture products in local markets, close to its customers. With plants in Europe, North America, South America and Asia, Gerresheimer generates revenues of around EUR 1.4 bn. The comprehensive product portfolio includes pharmaceutical packaging and products for the safe, simple administration of medicines: insulin pens, inhalers, micro pumps, prefillable syringes, injection vials, ampoules, bottles, and containers for liquid and solid medicines with closure and safety systems as well as packaging for the cosmetics Industry.
Gerresheimer AG
Klaus-Bungert-Straße 4
40468 Düsseldorf
Telefon: +49 (211) 6181-00
Telefax: +49 (211) 6181-295
http://www.gerresheimer.de
Senior Manager Group Communication
Telefon: +49 (211) 6181-246
Fax: +49 (211) 6181-241
E-Mail: m.stolzenwald@gerresheimer.com
Communication & Marketing Medical Plastic Systems
Telefon: +49 (9431) 639-6140
Fax: +49 (9431) 79838-6140
E-Mail: k.fischer@gerresheimer.com
![]()




