-
New Capital Strengthens ProtaGene’s Advanced Analytical Leadership in the Biologics and Cell and Gene Therapy Markets
ProtaGene, a leading global contract research organization (CRO) dedicated to advanced analytics for biologics and cell and gene therapies, today announced additional investment from Ampersand Capital Partners. Given the critical importance of advanced bioanalytics and product characterization for developing increasingly complex therapeutics, Ampersand has invested nearly $40 million within the past year to support ProtaGene’s services and capacity expansions. This investment has allowed the organization to meet rebounding market demand among biologics developers, address increasingly complex analytical challenges, and deliver the growing product understanding and safety assurances required by global regulatory bodies. Investments Fueled Capacity & Capabilities Expansions Ampersand’s investments have been pivotal in ProtaGene’s worldwide expansion efforts, including establishing…
-
Confronting Molecular Bioanalysis Challenges in Gene Therapy Development
As the gene therapy sector continues to mature, regulators’ expectations for more robust product understanding are growing. It’s exciting to be part of today’s gene therapy development community; however, three issues are at the top of the industry’s mind—the regulatory landscape, critical molecular bioanalysis considerations (biodistribution, transgene expression, and vector shedding), and assay-related challenges. #1 – Current Gene Therapy Regulatory Landscape Worldwide, there are more than 2,000 active clinical programs and thousands more molecules in various stages of pre-IND development, and the pace of commercial approval is accelerating. However, global regulators have applied more stringent requirements as the gene therapy market has matured. Validation requirements, especially for molecules in the…
-
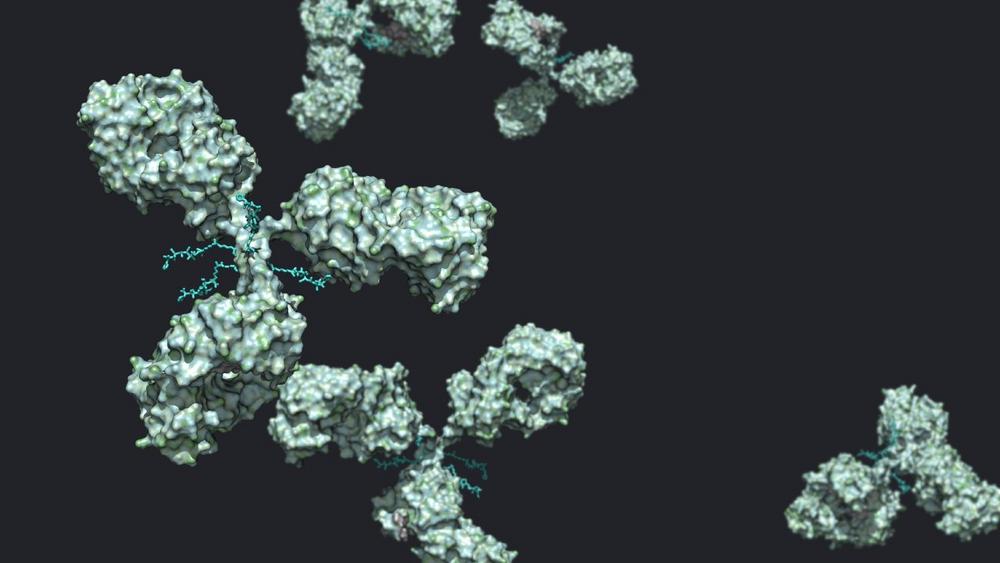
Navigating CMC Challenges in NBE and Therapeutic Antibody Development
In April 2021, the FDA approved the 100th monoclonal antibody as a therapeutic entity, a milestone that took 35 years to reach. As of the fall of 2022, the FDA has approved 162 antibody therapies, and approval momentum continues to accelerate. Classical IgG-based monoclonal antibodies (mAbs) are by far the most mature protein-based therapeutic modality, with a projected global compound annual growth rate of over 10 percent until 2030. However, newer antibody therapeutic constructs, like bispecific antibodies, antibody fragments, radiolabeled antibodies, and antibody conjugates, are gaining importance and are projected to experience compound annual growth rates as high as 44%. So what does all this growth mean for innovator organizations?…
-
ProtaGene Appoints New Scientific and Commercial Leadership to Support Evolving Needs of Biologic and Cell & Gene Therapy Innovators
ProtaGene GmbH, a leading global contract research organization focused on advanced analytics in biologics and cell and gene therapy, announced today the appointment of Jennifer Chadwick, Ph.D., as Chief Scientific Officer and Owen Hitchins as Chief Commercial Officer. Working in close collaboration, accomplished executive leaders Chadwick and Hitchins will utilize their respective experience to meet the rapidly evolving needs of the advanced biotherapeutics development community. "Innovators in today’s biopharmaceutical industry are working to bring increasingly complex products to market, which means greater challenges and unknowns along the development path where quality and regulatory expectations continue to evolve," said Chadwick. "As a strategic partner to numerous innovators, we provide solutions for…
-
Foundation for the Industry’s Next-stage Innovation and Growth
ProtaGene is thrilled to announce the planned opening of our brand-new, state-of-the-art lab and office headquarters in the Burlington BioCenter, located in Boston’s rapidly growing biotechnology hub. Our expanded footprint is designed to enable new capabilities required to advance today’s biologic and gene therapy platforms and provide additional capacity to meet the growing demand for our analytical services across all biotherapeutic sectors. The new facility will feature: – 27,000 square feet of brand-new Class-A lab and office space – triple the size of our current facility – Custom-designed protein and gene analytic labs, systems, and workflows – Centrally located Sample receiving, log-in, storage and monitoring – Facility and systems designed…